JACKSONVILLE, Fla. — As officials race to get shots in arms as quickly as possible and increase immunity to Coronavirus, scientists are closely watching how the vaccines already on the market will interact with newer strains of the virus.
Already, the United Kingdom strain, known as B.1.1.7., has begun to spread in the United States and is expected to become the dominant strain.
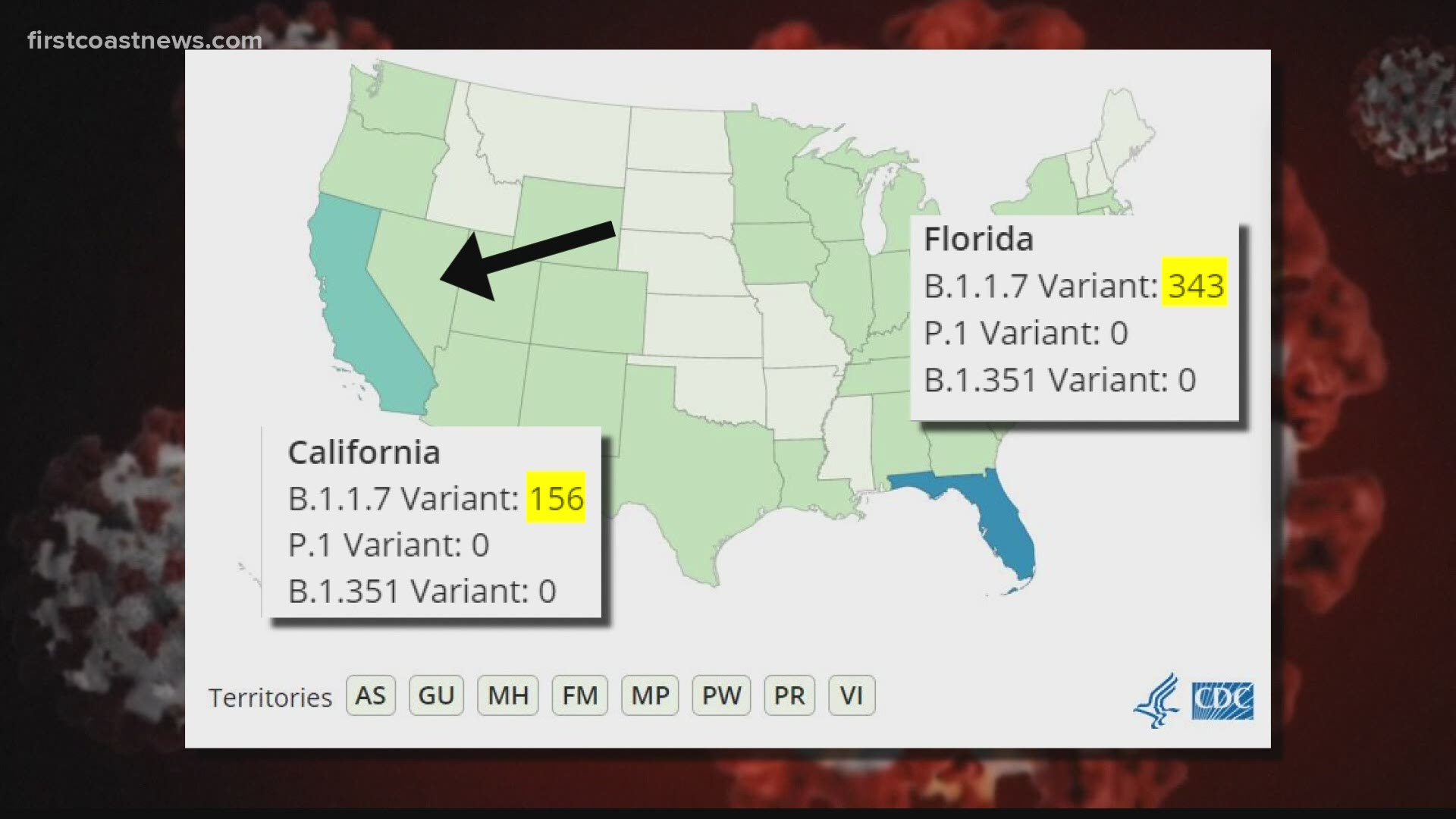
According to data from the Centers for Disease Control and Prevention (CDC), Florida leads the nation in confirmed cases of the variant, with 343 recorded as of Tuesday evening. The only other state that comes close is California with 156 confirmed cases.
However because of limitations on the ability to sequence every confirmed COVID-19 case to determine which are caused by the variant strain, experts say it is likely the U.K. strain is already widespread.
"There's a lot more [cases]," said Chad Neilsen, Director of Infection Prevention at UF Health Jacksonville. "We just don't have robust genetic sequencing in order to tell us that the variant strain is predominant."
Scientists agree the strain is more contagious and potentially more deadly, although studies are still being conducted.
Neilsen said that if the U.K. strain continues to spread at its current pace, it will become the dominant strain. Dr. Anthony Fauci said the same in January, predicting the U.K. variant could become dominant as early as March.
However for those who have already received doses of the Pfizer or Moderna vaccines, the only two currently approved for emergency use authorization by the Food and Drug Administration (FDA), experts say there is not necessarily a need to be concerned.
"You expect viruses to change during the course of an epidemic," said Dr. Michael Koren, Medical Director at Jacksonville Center for Clinical Research. "Be reassured that the vaccines that are now available are pretty darn effective still. Maybe not quite as effective as the original variant, but still really really good."
Koren said scientists are more closely monitoring vaccine effectiveness against a variant strain out of South Africa, known as B.1.351.
Currently, the CDC has only reported three confirmed cases of the South Africa strain in the United States, and none in Florida.
This week, South Africa halted the rollout of the AstraZeneca vaccine after a study showed "disappointing" effectiveness results against the new variant.
The AstraZeneca vaccine, which is one shot, has not shown the same effectiveness as the Pfizer and Moderna vaccines. Koren said it is likely nowhere near applying for FDA emergency use authorization.
Both Pfizer and Moderna use a newer technology called mRNA platform, which is not used in the AstraZeneca vaccine.
"They probably work better against the U.K. variant as compared to the South African variant," Koren said, adding that both Pfizer and Moderna still show high effectivity levels against the U.K. strain.
"Even people that get COVID-19 that have been vaccinated tend to have much milder cases," Koren explained. "So there seems to be some protective effect of just having your immune system exposed to the vaccine, even if you end up testing positive for COVID-19."
Neilsen said it's now a race to spread immunity to the virus through vaccinations. Already, at least temporary immunity is spread when a person contracts COVID-19 and recovers.
"If the virus doesn't have a host to infect, it doesn't have a host to mutate in," Neilsen said. "The longer that it takes for us to get the vaccine out to the general population, the more people the virus can infect and in fact mutate in."
If mutations continue to arise and begin to impact the effectiveness of the vaccines currently on the market, Neilsen said it would not be difficult to alter the Pfizer or Moderna mRNA vaccines to adapt.
"If we continue to see mutations, if these mutations can evade the vaccines, we will have to start reformulating the vaccines and we could be looking at the potential for an annual vaccine, we just don't know yet," Neilsen said.
Meanwhile, pharmaceutical company Johnson and Johnson is expected to receive emergency use authorization from the FDA for its one-shot vaccine this month.
If the J&J vaccine is approved, it would be the third vaccine to be distributed in the United States and could ramp up the speed of distribution.