JACKSONVILLE, Fla. — Americans could find out Thursday if the FDA will approve emergency use of a coronavirus vaccine in the United States.
Doctors at the Mayo Clinic in Jacksonville say they are optimistic about the vaccine being released in the country. They say for most people in trials side effects include mild fever, headaches and body aches.
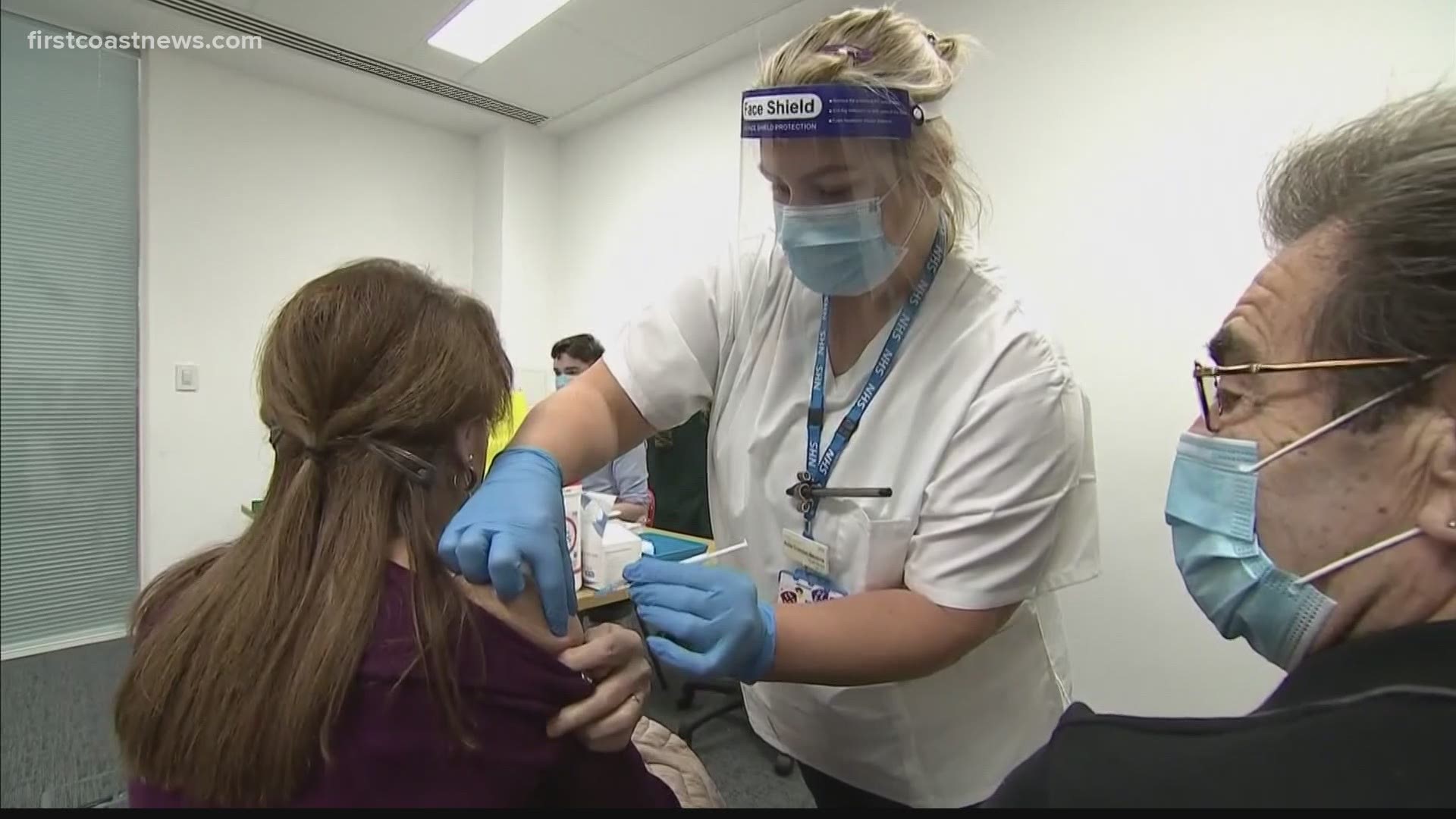
Across the Atlantic in the United Kingdom, the Pfizer COVID-19 vaccine has already been released. Thousands have already received the first dose of the vaccine.
“I did not think that we would be here by December," said Dr. Abinash Virk, a pediatric infectious disease specialist with the Mayo Clinic.
Virk believes the Pfizer and Moderna vaccines, which the FDA is considering for emergency use authorization, are safe overall.
“Yes, they both have some degree of what I call mild to moderate symptoms with a reaction to the vaccine, but these are not severe, adverse reactions," Virk explained.
Two people who received the vaccine in the United Kingdom had a severe allergic reaction. Virk says both patients had underlying health conditions.
“When we vaccinate people, we are always prepared for anybody to have an anaphylactic or an acute reaction right away," Virk said.
Dr. Melanie Swift says people who have been vaccinated should still social distance and wear a mask.
“We can’t just rip off our masks just because we’ve had our vaccine until we know for sure that means we’re not having asymptomatic disease that we could spread," said Swift, who works in occupational medicine at the Mayo Clinic.
Swift also said it is imperative that people get the vaccine to help quell the spread of the virus.
“We can’t rely on partial vaccination coverage," Swift said. "In order to stop transmission, we have to have widespread immunity.”
The FDA is expected to look at emergency use authorization for the Pfizer vaccine Thursday and the Moderna vaccine on Dec. 17.
Virk says pregnant women should not get the vaccine because they were not included in any studies.